Abstract
Introduction
Post-transplant cyclophosphamide (PTCy) is widely used for graft-versus-host disease (GVHD) prophylaxis after haploidentical hematopoietic stem cell transplantation (SCT). However, the optimization regarding the doses of PTCy and the schedules of calcineurin inhibitors has not been well studied in a clinical setting. To investigate the clinical impact of these factors, we retrospectively analyzed 61 patients who received allogenic stem-cell transplantation with a related-haploidentical donor as grafts of peripheral blood with the GVHD prophylaxis of PTCy/Tac/ mycophenolate mofetil (MMF) at our center from January 1, 2016 to June 30, 2022.
Methods
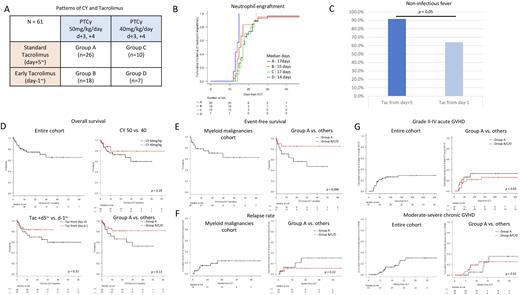
In total 61 patients, the median age was 52 years and 29 patients received myeloablative conditioning (MAC) regimen. Myeloid malignancies (MNs) were the most frequent indication for transplantation (n=39). We divided the whole cohorts into 4 groups as follows; group A) the standard dose of PTCy (50mg/kg/day, day +3, +4) with standard administration of Tac (day +5~) (n=26), group B) the standard dose of PTCY (50mg/kg/day, day +3, +4) with early administration of Tac (day -1~) (n=18), group C) the reduced dose of PTCy (40mg/kg/day or less, day +3, +4) with standard administration of Tac (day +5~) (n=10), and group D) the reduced dose of PTCy (40mg/kg/day or less, day +3, +4) with early administration of Tac (day -1~) (n=7) (Figure A). All patients received MMF from day +5.
Results
No statistical differences in the total median days of Tac and MMF administration in each group were observed (Tac; 6.2, 8.1, 8.7, and 6.6 months, and MMF; 37, 37, 37, and 31 days, in group A, B, C, and D, respectively).
The median time for neutrophil and platelet engraftment was earlier in group B and D (early Tac group) than group A and C (standard Tac group) (neutrophil; 15 and 14 days vs. 17 and 17 days, p = 0.003, Figure B, platelet; 20 and 19 days vs. 24.5 and 25 days, p = 0.28). Patients in group B and D (early Tac group) were significantly less likely to have a non-infectious fever before PTCy administration than those in group A and C (standard Tac group) (64.0% vs. 91.7%, p = 0.01, Figure C).
The median and 1-y overall survival (OS) from transplantation to death or last follow-up was not reached and 77% (95% confidence interval [CI]; 63.6-86.0%, Figure D). Patients in group A and B (50mg/kg/day) or in group A and C (standard Tac group) had lower 1-y OS than those in group C and D (40mg/kg/day) (75.9% in group A and B vs. 79.1% in group C and D, Figure D) or in group B and D (early Tac group) (72.7% in group A and C vs. 83.6% in group B and D, Figure D), respectively. Thus, patients in group A had lower 1-y OS than the set of the other three groups (71.0% vs. 81.3%, p = 0.13, Figure D). In contrast, no differences of 1-y OS were observed in patients with MAC (73.9%) and reduced intensity conditioning (RIC) regimen (79.3%). In terms of event-free survival (EFS), defined events as relapse, death, or last follow-up, in patients with MNs, 1-y EFS was 78.4% (95%CI; 61.4-88.6%, Figure E). As well as OS, patients in group A had almost significantly inferior 1-y EFS than those in the other group set (70.6% vs. 85.2%, p = 0.096, Figure E). However, no difference of 1-y EFS was observed in patients with MNs according to the intensity of the conditioning regimen.
The cumulative 1-y and 2-y relapse rate in patients with MNs was 16.7% and 23.2% (Figure F). As well as the EFS and OS, patients with MNs in group A had higher cumulative 2-y relapse rate (30.1%) than those in the other group set (10.6%, p = 0.18) (Figure F).
The cumulative rate of 100-day Grade II-IV acute graft versus host disease (GVHD) and 2-y moderate-severe chronic GVHD in all patients were 25.9% and 30%, respectively (Figure G). Patients in Group A had similar incidences of acute and chronic GVHD compared to those in the other group set (Grade II-IV acute GVHD at day 100; 33% vs. 22.8%, p = 0.5 and moderate-severe chronic GVHD at 2-y; 35.2% vs. 25.3%, p = 0.52, Figure G).
Conclusion
Our preliminary results suggest that a higher frequency of death due to disease recurrence in group A (PTCy; 50mg/kg/day, day +3, +4 with standard administration of tacrolimus) than in the modified PTCy group, while the incidences of acute and chronic GVHD were comparable. Patients with the potential risk of relapse may prefer a reduced CY dose or an early administration of Tac. Further analyses to validate our results are warranted.
Disclosures
Asada:Asahi KASEI: Speakers Bureau; Abbvie: Speakers Bureau; Astellas: Speakers Bureau; Kyowa KIRIN: Speakers Bureau; Meiji: Speakers Bureau; Nippon Shinyaku: Speakers Bureau; Novartis: Research Funding, Speakers Bureau. Ennishi:Nipponshinyaku Pharmaceutical Co., Ltd.,: Research Funding; Chugai Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Eisai Pharmaceutical Co., Ltd.,: Honoraria; Kyowa Kirin Pharmaceutical Co., Ltd.: Honoraria. Maeda:Novartis Pharma: Research Funding, Speakers Bureau; Nippon Shinyaku: Honoraria, Research Funding; Chugai Pharmaceutical: Honoraria, Research Funding; Astellas: Honoraria, Research Funding; AstraZeneca: Research Funding; Takeda: Honoraria; Kyowa Kirin: Honoraria; Eisai: Honoraria; Otsuka Pharmaceutical: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal